
Introduction
Both quinoa production and consumption worldwide have been growing exponentially. As an example, quinoa cultivation has increased from seven countries in 1980 to 123 countries in 2018. Despite the rapid expansion of this crop, countries such as Peru and Bolivia continue to supply 74% of the world market for this grain. Nevertheless during the last decade the production prices of quinoa collapsed, while the yields remained low, so it is necessary to propose local strategies that are based on the diversity of cultivars, their incorporation of food and cooperation to favor its consumption (Alandia et al., 2020).
This situation is gaining importance in Colombia, where recent studies lacked diversity in the genetic material present and cultivated in the country (Guerrero-López, 2018). However, because of non-incorporation of quinoa in the annex list of the Convention on Biological Diversity held in Rio de Janeiro, its seeds began to be used in different parts of the world, which changed its biological behavior when established under different agroecological environments (soil, rainfall, temperature, and altitude; Ruiz et al., 2014). This situation also occurs in Colombia, where there are more than 30 accessions different in genetic, morphological, and productive attributes (Manjarres-Hernández et al., 2021).
Quinoa diversity analyses have been conducted under controlled or cold climate conditions and lack information related to compositional characteristics of the grains (Torres et al., 2000; Delgado et al., 2009; García-Parra et al., 2019). For this reason, Reguera et al. (2018) highlights the importance of evaluating quinoa cultivars through the interaction between environment and genotype for more information, leading to a complete analysis of this species. However, the information that reports the effect of the environment on the compositional characteristics of quinoa grains is clear and mainly on lipid, protein, starch, fiber, and bioactive compound structures (Miranda et al., 2013; Curti et al., 2019; Matías et al., 2022).
One of the climatic factors with greatest impact on the productive performance of plants is altitude, as it brings together climatic elements associated with radiation and temperature (Haeberli and Alean, 1985). Temperature is crucial for active and passive transport between source and dump organs, as well as their structuring in fruits (Smith et al., 2018). Such a situation is vital in photoassimilate transport rate, as well as in transport pathway, for which plant seeds conform into protein, lipid, and starchy structures and depend on individual genetic basis and the environment, as well as the transport pathway developed in relation to the movement of photoassimilates, as highlighted by Lee et al. (2019) in rice plants.
In this sense, Thiam et al. (2021) present results of the environment-genotype interaction in quinoa cultivars and find a strong influence of climatic elements on grain yield parameters. The previous results were similar to those obtained by Granado-Rodríguez et al. (2021), who determined that the environment-genotype interaction modifies the nutritional response of quinoa grains. Also, García-Parra et al. (2021) made the first report on the effect that genetic variation (cultivars) has on the quantity and secondary structure of starch (amylose and amylopectin ratio) and proteins (β-sheets 1 and 2, random coil α-elice, and β-turns 2 and 3) present in quinoa grains.
This phenomenon results from the biological and physicochemical responses of quinoa grains (Bertero et al., 2004; Stikic et al., 2012); however, most of the studies have advanced in the effect that different industrial processes have on the grains, but they forget the influence the environment-cultivar interaction has by contributing in the chemical and phytochemical characteristics of quinoa seeds (Antognoni et al., 2021; Granado-Rodríguez et al., 2021). For this reason, this study aims to find an effect of agroclimatic conditions on the physicochemical and rheological characteristics of different quinoa cultivars in the Department of Boyacá-Colombia.
Materials and Methods
Study Area
The study areas are located in the department of Boyacá in three regions of central Colombia in 2020, established under three altitude gradients corresponding to cold, temperate, and warm climates according to the Caldas-Lang methodology (Cantillo and Gracia, 2013; Figure 1). Quinoa seeds of cultivars Blanca Real, Nariño, Pasankalla, Puno, and Titicaca were collected to evaluate agro-industrial potential traits in three areas with altitudinal gradient conditions: Oicatá (5° 36′N-73°10′W; 2,648 ma.s.l.), Moniquirá (5° 52′N-73° 33′W; 1,707 ma.s.l.), and San Pablo de Borbur (5° 39′N−74°04′W; 698 ma.s.l.). The field experiments in Oicatá, Moniquirá and San Pablo de Borbur were carried out under rainy conditions between April and August 2020 with 421-, 718-, and 1,043-mm total precipitation during the mentioned period.
Figure 1. Study area. Department of Boyacá, Colombia, South America. Climatic conditions of the three study areas.
The type of soil in Oicatá is an association intro Vertic Haplustalf–Andic Dystrustepts, with pH 5.8, organic matter 4.1%, and interchangeable bases (in cmol/Kg) of Ca, Mg, K, and Na equivalent to 2.5, 1.12, 1.07, and 0.14, respectively. The type of soil in Moniquirá is an association Humic Dystrudepts-Typic Dystrudepts with pH 4.9, organic matter 6.1%, and interchangeable bases (in cmol/Kg) of Ca, Mg, K, and Na equivalent to 2.2, 0.7, 0.7, and 0.1, respectively. The type of soil in San Pablo de Borbur is an association intro-Typic Eustrudepts–Typic Udorthents–Humic Dystrudepts with pH 4.1, organic matter 3%, and interchangeable bases (in cmol/Kg) of Ca, Mg, K, and Na equivalent to 1.5, 0.3, 0.3, and 0.02, respectively (IGAC, 2005). The experimental design consisted of plots of 9 m2 (3 × 3 m) with 6 grooves and 8 Kg. ha−1 of planting density. Each cultivar was established with three replicates. The seeds were collected from plants that grew in the center of the experimental unit (2 m2), neglecting plants from the perimeter area of the plot (7 m2). These were harvested in the phase of physiological maturity, harvesting grains from the middle third of the panicle. The grains were manually cleaned for subsequent analyses.
Proximal Characteristics
To determinate protein content, the Kjeldahl method was used. AOAC 960.52 was multiplied by a conversion factor of 6.25 to measure total raw protein from the seeds. Fats were defined with the AOAC 922.06 method using Soxhlet (Soxtec 2050). Finally, total carbohydrates were calculated by the difference (i.e., protein + ash + fat + moisture – 100) (AOAC, 2016).
Mid-Range Infrared Spectroscopy FT-IR
IR spectrum was obtained using the IRAFFINITY-1S equipment (Shimadzu Corp., Kyoto, Japan). The spectrum was obtained by the reflection mode between 400 and 4,000 cm−1 and proportional over 32 scans with 4 cm−1 and a temperature of 25°C. In previous measurements, each cultivar had a blank background spectrum. A spectrum analysis was conducted using the 7th version of OriginPro. The measurements were fixed and standardized over a baseline between 0 and 1 (represented in the figures) to show the highest transmittance peaks. Deconvolution was performed on a spectrum using Fourier transform to determine protein secondary structure changes at 1,600 and 1,700 cm−1 (García-Parra et al., 2021). Later, modeling of the Gaussian function spectrum was applied. Starch concentration was studied from its formation on a short-range band between 875 and 1,175 cm−1. Lipids were present on the 2,800 and 2,900 cm−1 range (Roa-Acosta et al., 2020). All samples were analyzed in triplicate.
Flow Properties
The flow properties of a 12% dispersion were determined using a rheometer (TA Instruments, R 1500, New Castel, United States, equipped with a concentric cylinder geometry. Shear rate was increased from 0 to 300 s−1 over 5 min. Average apparent viscosity was determined between 180 and 200 s−1, avoiding the transition zone because of shear rate (Polo et al., 2021). Flow curves (shear stress vs. shear rate) were obtained and fitted to the power law model (Equation 1).
where τ is the shear stress (Pa), γ is the shear rate (s), K is the consistency coefficient (Pa.s), and n is the flow behavior index. The flow behavior index indicates Newtonian (n = 1), shear thinning (pseudaplastic, n < 1), and shear thickening (dilative, n > 1) flow behaviors. Consistency and flow were determined before and after the pasting curve in order to determine the effect of thermal processing on these parameters. Finally, the data were smoothed using the Savistky-Golay function in the GraphPad Prism version 6 software.
Viscosity Profile
Pasting properties were measured in a rheometer (TA Instruments, AR 1500, New Castel, United States). A flour dispersion (12% w/v) was prepared by adding the powder to distilled water and suspending at room temperature for 1 min, and angular velocity (78.53 rad/s) in the rheometer cylinder. To determine the pasting properties, the rheometer was programmed to run temperature sweeps consisting of heating (25–90°C), tempering (90°C for 5 min), and cooling (90–25°C) steps at 10°C/min. Viscosity peak, breakdown (BD) viscosity, and set back (SB) viscosity were recorded in duplicate (Polo et al., 2021).
Statistical Analysis
The analysis comprised six cultivars of quinoa established under three different altitude gradients, for which a completely randomized design with a 6 × 3 factorial arrangement was established where the first factor comprises cultivars and the second areas where the grains were grown and harvested. The assumptions of normality and homogeneity were evaluated by the Shapiro–Wilk and Bartlett tests. The multiple analysis was compared with the Tukey HSD test with a level of significance p ≤ 0.05. Correlations were established between the quantitative variables using the Sperman correlation and were plotted using the corrplot library. A double-grouping analysis was performed by Manhattan distance and cluster method by Ward with the gplots and RColorBrewer libraries. The data were analyzed in statistical software R version 3.6.1.
Results
Proximal Analysis of Grains
The data associated with the proximate analysis of protein, carbohydrate, and fat contents showed statistical differences among treatments, being significantly high in relation to the altitudinal gradient (p < 0.05) and cultivar (p < 0.001), while for the interaction (p < 0.01), there were no differences in carbohydrate content. In the percentage of protein, there was high heterogeneity in its content, where the Titicaca cultivar presented the highest value in the cold climate altitudinal gradient (Table 1).
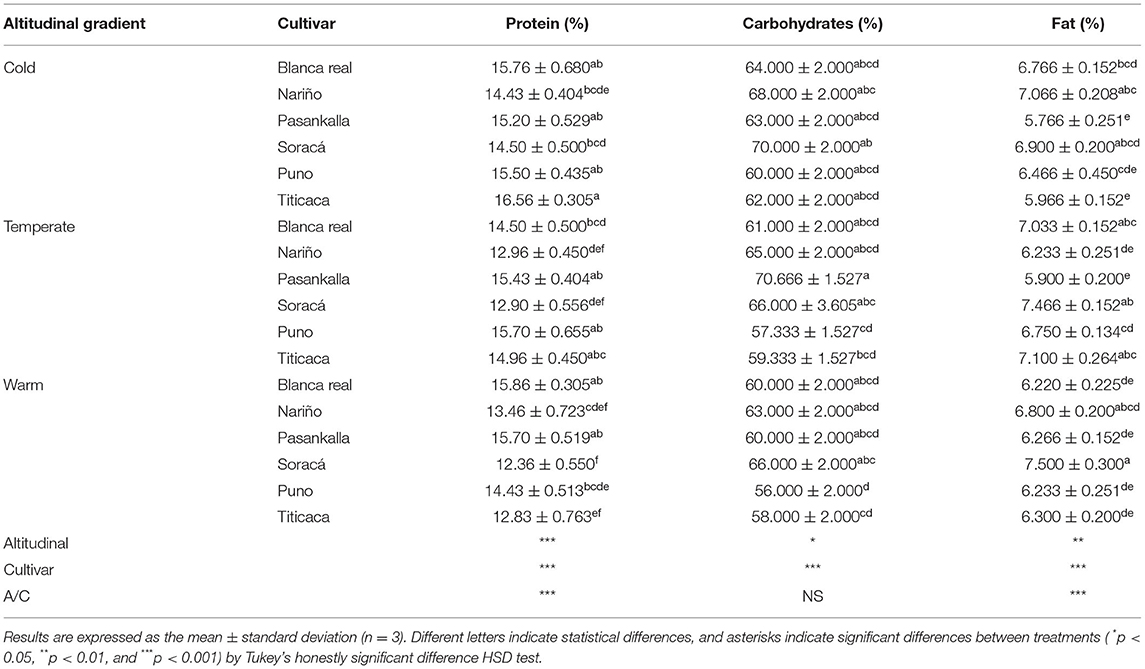
Table 1. Proximate characteristics of six quinoa cultivars under three different altitudinal gradients.
In the same sense, the cold climate cultivars presented, on average, the highest protein value (15.32%) compared to the temperate (14.41%) and warm (14.11%) climate cultivars. With carbohydrates, the Pasankalla cultivar in the temperate climate contained the highest amount, while the highest content was found in the cultivars in cold climate (65.5%), followed by temperate climate (63.22%), and warm climate (61.44%). The volume of fat in the grains varied according to the climate of the established cultivars, since the highest values were found in the temperate climate (6.74%).
FT-IR Spectroscopy
FT-IR spectroscopy studied the bands of higher transmittance in different-treated quinoa grain flours in complete spectra with scans between 400 and 3,000 cm−1. The N-H bending and C-N stretching in the 1,547 cm−1 band showed significant differences for the treatments with higher intensities in the cultivars in cold (0.7 ± 0.06) and temperate (0.71 ± 0.08) climates. Thus, the C=O stretch measured in the 1,634 cm−1 band joined the climates in cold and warm groups as the cultivars with the highest peak intensity and temperate climate as those with the lowest, while the highest intensity for the two bands occurred in the cultivar Blanca Real in warm climate (Table 2). In C-O stretching, and C-O-C and C-O folding in the 1,015 cm−1 band, quinoa grains produced under cold climate conditions presented the highest transmittance peak, with Puno intemperate climate having the highest peak intensity. In addition, the C-H stretch in the 2,923 cm−1 region showed significant differences among cultivars, climates, and their interaction (p < 0.001), with Pasankalla being the cultivar with highest intensity in the cold climate.
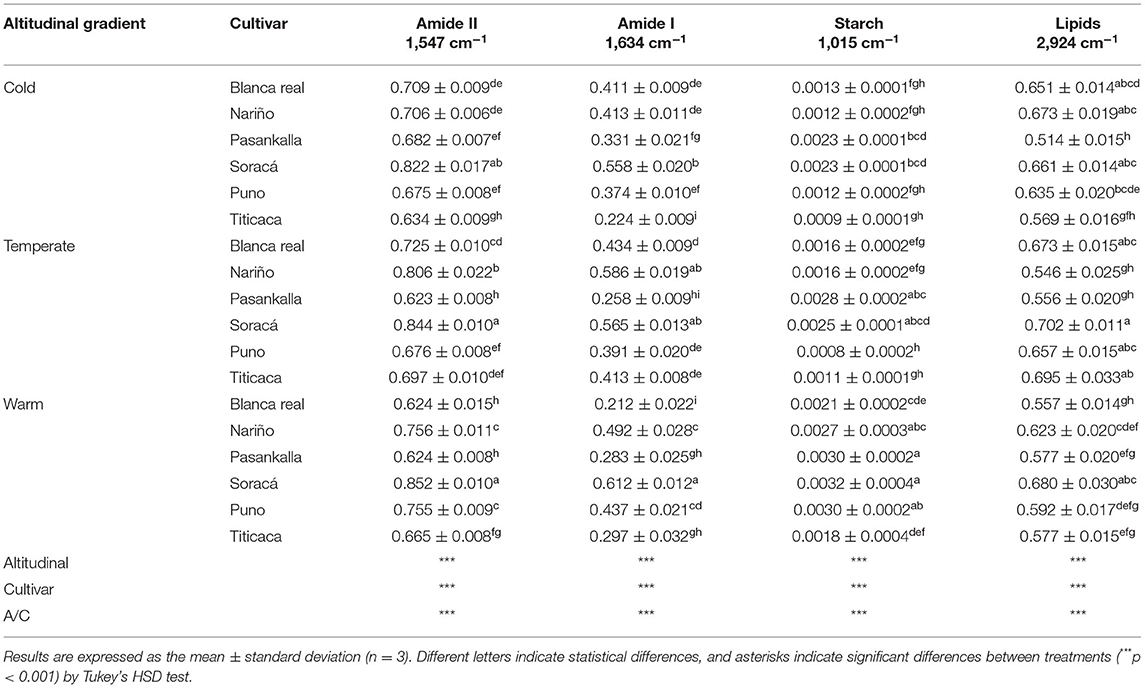
Table 2. Intensity (a. u.) of bands associated with structures of interest in grains of different quinoa cultivars.
Secondary Protein Structure
In this study, the secondary protein structure of different flours of quinoa was analyzed with the FT-IR analytical technique. For this purpose, the different flour cultivars produced changes in intensities of the characteristic regions of protein-related regions of amides I and II. The results allowed for identifying significant statistical differences among the cultivars, climates, and their interaction (p < 0.01) except for a random secondary protein, which did not show any significant difference (Table 3).
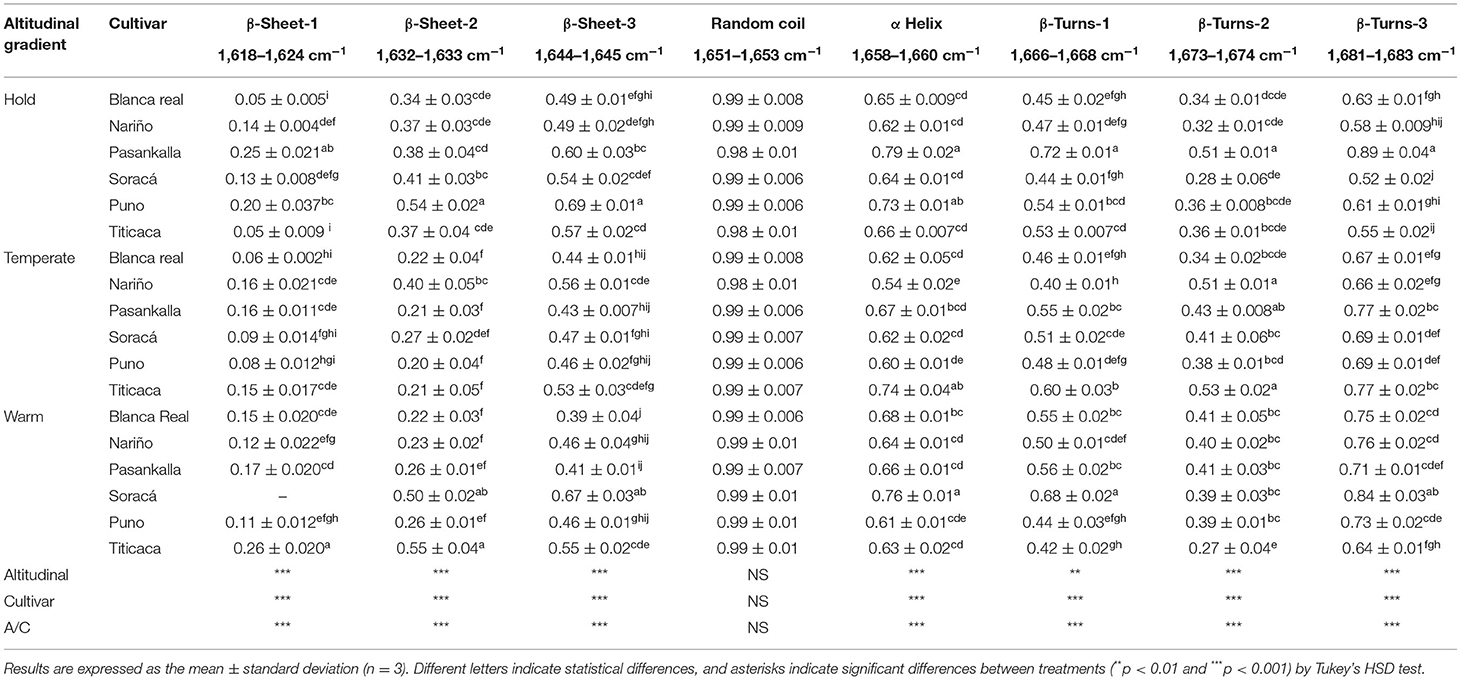
Table 3. Intensity (a.u) of secondary protein structures of diverse quinoa cultivars in three different altitudinal gradients.
Structural Order of Starch
In contrast to the secondary structure of protein, the structural organization of starch was not affected by altitudinal gradient but by the difference between the cultivars used. The most intense peak corresponded to 1,075 cm−1, while the cultivars with best peaks were Blanca Real and Pasankalla in the regions 995, 1,022, and 1,047 cm−1 (Table 4). Glycosidic band 1,075 cm−1 was associated with starchy content by C-O-C vibrations functional group, despite not finding differences intro of the cultivars and altitudinal gradient.
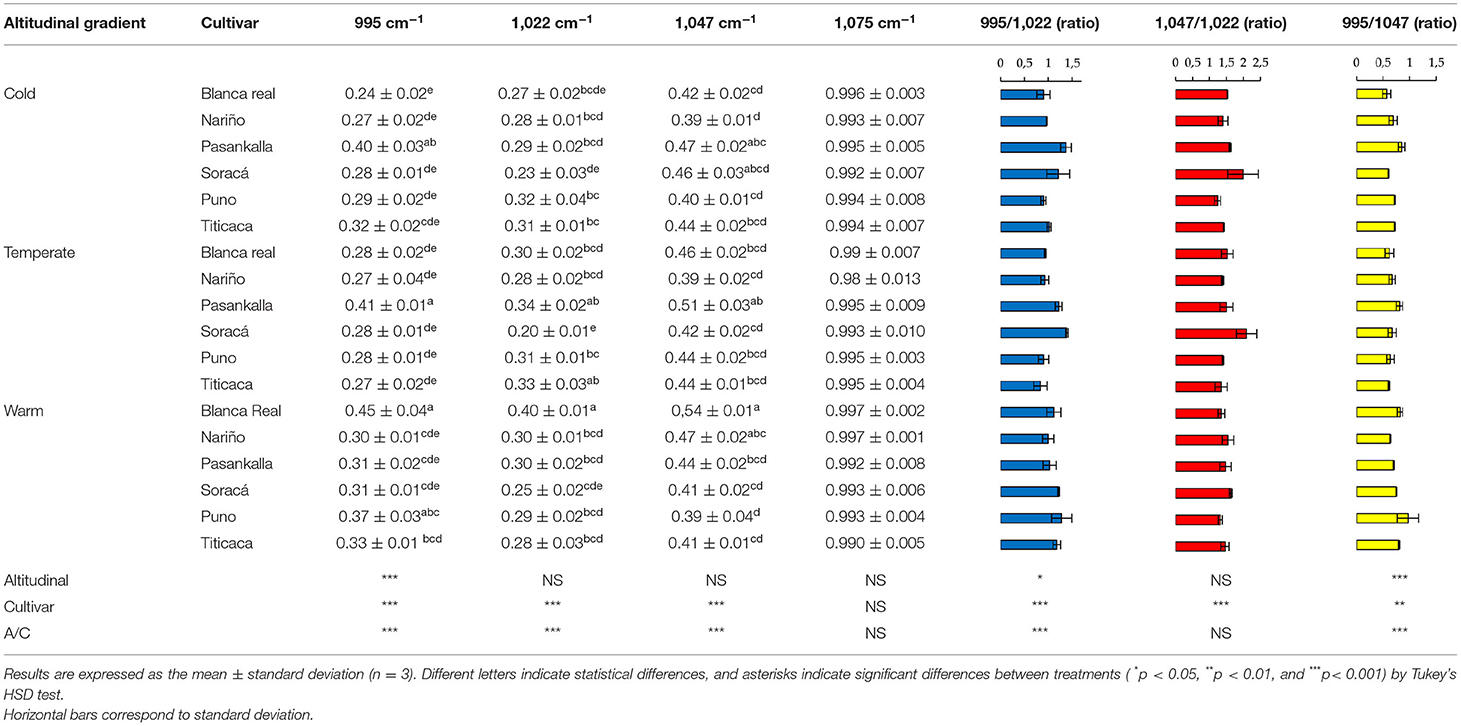
Table 4. Intensity (a.u.) of Fourier transform infrared (FT-IR) spectra of starch region in different quinoa cultivars.
Flow Properties
The results showed significant statistical differences between treatments under the dispersion of 12%. As shown in Table 5, the highest viscosity peaks were 1.09 and 1.13 Pa s−1 between the warm and cold climate Soracá cultivars. This increased viscosity occurs during the heating phase, which happens when gelatinization occurs in starch granules. However, the difference among the viscosity peaks is due to the molecular conformation of starch and proteins in the grains of the cultivars. Compositional differences between the same species are characteristics associated with genetic diversity and influence of the soil and climatic environments, because they determine the ratio of starch to protein.
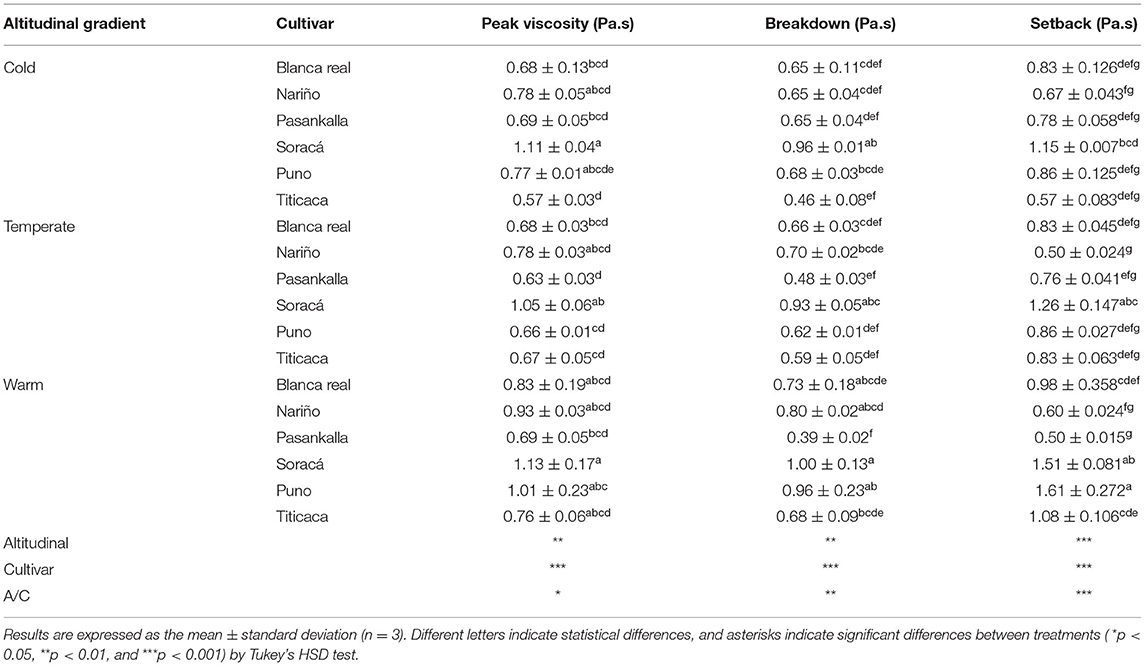
Table 5. Pasturing parameters of different quinoa cultivars grown under three different altitudinal gradients.
Table 6 shows the viscosity behavior before and after heating/cooling under different stress conditions (Pa) and shear rates (s−1). The coefficients were obtained by regression of the power model where n is the flow rate and K is the consistency. The dispersion of each treatment underwent a variation in shear rate from 0 to 100 s−1, observing its effect on viscosity.
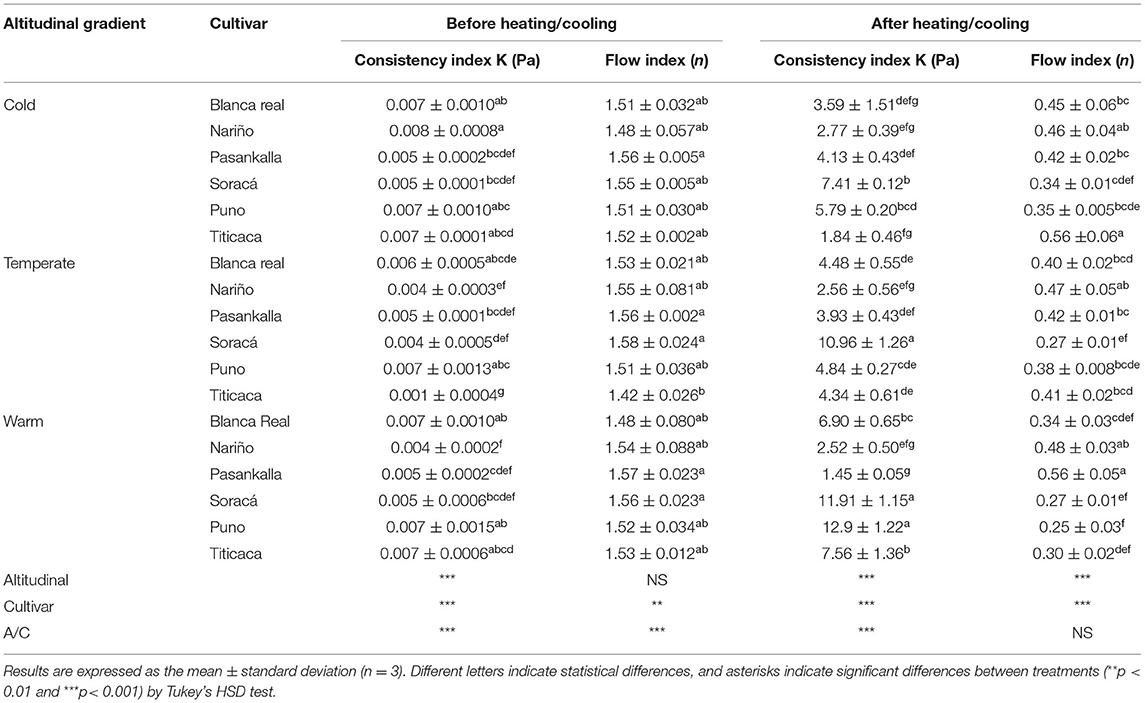
Table 6. Behavior of quinoa flours at shear rate on flow rate and consistency.
According to the results, there are significant changes before and after heating/cooling. All the flours presented a dilatant behavior before heating/cooling with flow indexes between 1.58 and 1.42. However, the flours gained a pseudo-plastic behavior that varied between 0.25 and 0.56 after the dispersion experienced the thermal cycle, i.e., the flow indices tend to have values lower than 1.
Discussion
Quinoa is often compared to cereals, since its characteristics are similar in composition and use. However, its agro-industrial potential is not yet reached under new cultivation regions, with deficiencies in research that relates primary production with the agro-industrial characteristics of grains compared mainly to crops such as maize, rice, and wheat (Stikic et al., 2012). The establishment and adaptation of quinoa cultivars to these new edaphoclimatic contexts can result in changes in nutritional properties of quinoa grains, which are associated with variations in the genotype, the environment, and their interaction (Granado-Rodríguez et al., 2021).
As previously described, the results show a changing composition in the proximal allocation of seeds, which ratifies the effect that climate and its relationship with the genetic variation of the species have on the potential use of grains. This is evidenced in other cultivars and under tropical and extratropical conditions compared by Reguera et al. (2018) who highlight the effect of climate, soil, water, fertilization, establishment, and management practices, as well as quinoa genotype. Thus, the production of four quinoa cultivars at two different sowing times affected the protein concentration in the grains, which is attributed to changes in precipitation and temperature during the test periods (Präger et al., 2018).
In quinoa, the effect of edaphic nitrogen and radiation on the characteristics of quinoa grains has been reported (Bascuñán-Godoy et al., 2018; Eustis et al., 2020), and is mainly attributed to the effect of temperature environmental and the function of mineral elements on the exchange rate between CO2 and water vapor, its incorporation in the carbon fixation phase and the ability to produce triose phosphate molecules for storage as starch in the chloroplasts or transported to the cytosol as hexoses that initiate the structuring of the reserve organs, mainly the perisperm where the greatest part of carbohydrates (Lemoine et al., 2013). In addition, it has been reported that in the anthesis phase, the transport of hexoses and amino acids to ovule cells is regulated by the characteristics of the abiotic environment, being able to change from symplastic to apoplastic transport, determining the compositional characteristic of the grains and being able to modify the concentration of proteins, carbohydrates, lipids, and water in grains (Zúñiga-Sánchez et al., 2017).
The secondary structure of quinoa has not been well-studied by deconvolution spectroscopy. However, García-Parra et al. (2021) and Roa-Acosta et al. (2020) identified structures such as β-sheet, random coil, α-helix, β-turns, and β-type, between the bands 1,600 and 1,700 cm−1, being able to change the intensity of the peaks of these structures because of the effects of genetic diversity, agro-industrial processing, or climatic factors during primary production, as manifested for other species (Nawrocka, 2013; Li et al., 2020; Pezzotti et al., 2021). Table 2 shows that the 1,547 and 1,634 cm−1 bands associated with amides II and I present a higher peak intensity in the cold and warm climates, responding to the influence generated by the environmental temperature and hydric status on the speed of gas exchange, transport of substances, and their discharge in storage organs; such a phenomenon is very variable in plants that present C3 metabolism (Sonnewald et al., 2020), because the stomatal opening determines the speed of multiple physiological activities and accumulation of proteins in the seeds. A similar situation occurs in the 1,015 cm−1 band associated with starch, the highest proportion of which was found in cultivars in cold climates, which is a natural response of the species, since its ideal abiotic condition brings together climatic elements of cold zones (Torres et al., 2000).
Although some studies report that the amount of proteins is not affected by edaphoclimatic factors such as temperature, edaphic fertilization, and even growth season (Lesjak and Calderini, 2017; Präger et al., 2018; García-Parra et al., 2020), it has been found that the molecular organization of proteins can change, affecting their organization and functionality, as affirmed by Wang et al. (2020), who identified four secondary protein structures in six varieties of Chinese quinoa present in bands like those found in this study. However, both the intensity of the peaks and the band where the characteristic bonds of each structure vibrate or flex may change because of the variety used, characteristics of the production area, and management practices in primary production.
As production climate is a determining factor in the structural configuration of proteins, it is necessary to emphasize that not only the agro-industrial processes developed with the grains can change their techno-functional characteristics but also that performance during the growth and development of the grains can modify their compositional characteristics. In this sense, Wolkers et al. (1998) found that the maturation speed in seeds changes the intensity of the peaks of the secondary protein structure, especially in α-helix and β-sheet, which increased when presenting slower maturation periods, becoming an additional factor of evaluation for the grains of different quinoa cultivars together with the climate where they are grown and the speed of maturation.
However, the changes were not only given in the protein structure but also in the starchy conformation of the grains. Therefore, the band corresponding to 1,047 cm−1 is associated with crystalline structures, the 1,022 cm−1 is attributed to amorphous structures in starch, which is the reason the different ratios between amorphous and crystalline structures influence the order in this fraction of the grains, while the 995 cm−1 region is given to bending and vibration of C-OH bonds (Ahmed et al., 2018). However, the functionality of starch in food matrices has been attributed mainly to species, ignoring the effect of climate as reported by Labuschagne et al. (2007) and Yu et al. (2015) when establishing its effect on amylose/amylopectin ratio in grains. Thus, an example of its importance lies in its use to obtain gelled or translucent films, given that a high crystallinity index is required, while its incorporation into nutritional matrices requires starchy structures with low crystallinity indexes, since it reduces the retrograde phenomenon (Selma-Gracia et al., 2020).
Among the cultivars in diverse climates, those grown in warm climates showed, on average, higher peak viscosity compared to those grown in cold and temperate climates. This phenomenon can be attributed to environmental conditions, even though starch biosynthesis is regulated by genetic expressions of the species. It has been reported, that changes in photosynthetic rate due to effects on the inhibition of electron transport in photosystems during fruit growth periods alter the activity of starch-sitases (Tofiño et al., 2006), that of GBSSI that synthesizes amylose (Raynaud et al., 2016). In addition, inactivation of the enzyme ADP-glucose pyrophosphorylase induces increase in amylopectin content and transport of proteins associated with perisperm plastids, changing the kinetic properties of the kernels (Tetlow et al., 2004), since proteins cannot gelatinize like starch.
In this sense, the same cultivars with highest viscosity peak presented highest values in the setback, which is associated with high amylose contents in the perisperm matrix of quinoa grains, obtaining gels with higher water absorption capacity within the free linear and helical structures, as hydrogen bonds capture water molecules by reorganizing and generating gels with high viscosity peaks (Solarte-Montúfar et al., 2019).
Correlation Analysis
Relationships between the variables evaluated are within the differences given by the production place of the quinoa grains, the cultivars used, and their interaction. Producing quinoa under cold climate conditions creates strong relationships among peak viscosity, breakdown, and setback. In addition, there is an inverse relationship between consistency before and after heating/cooling with flow rate before and after heating/cooling.
The absorbance of the 995/1,022 cm−1 band has a positive relationship with consistency before heating/cooling, while the protein content and absorbance of amides I and II show an indirect relationship with viscosity peak and a direct relationship with the volume of carbohydrates. For the cultivars in temperate climate, there was a significant association between consistency after heating/cooling with setback and peak viscosity, and an inverse association with flow rate after heating/cooling. For the 995/1,022 and 1,047/1,022 cm−1 ratio bands, there was a significant relationship with viscosity peak. As in the cold climate, negative relationships were identified with the absorbance of amides and protein content.
Quinoa cultivars in warm climates showed a significant correlation among peak viscosity, breakdown, and setback, and there was a positive relationship between consistency after heating/cooling and setback, and a negative relationship with flow rate after heating/cooling. As in the cultivars of the other climates, there was an inverse relationship with the absorbance of amides I and II, and a positive relationship with carbohydrate content (Figure 2).
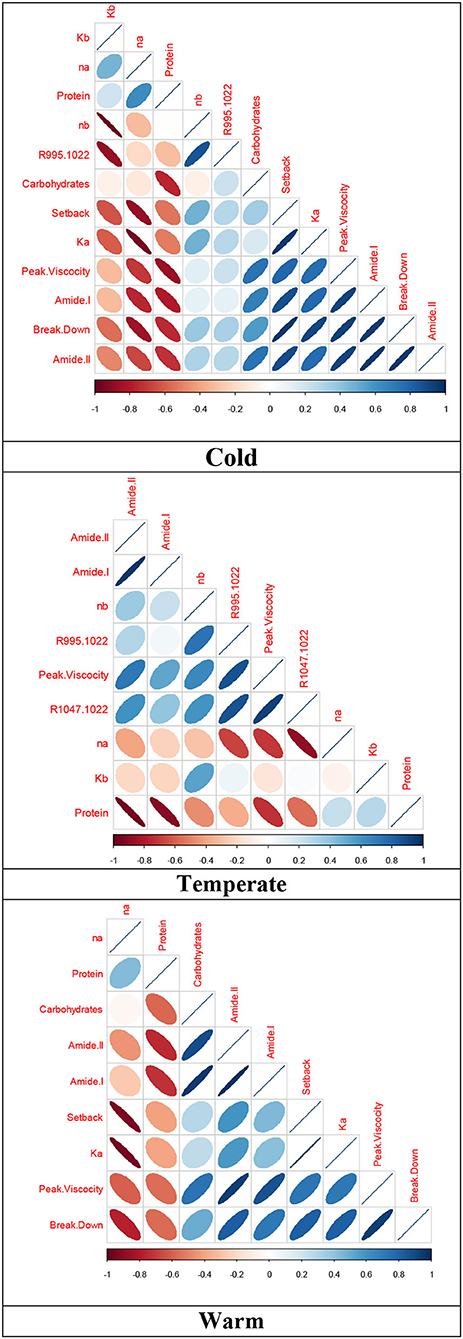
Figure 2. Spearman’s correlation for structural and rheological attributes of quinoa grains harvested at different altitudinal gradients. Red cells indicate negative correlations, and blue cells show positive correlations.
According to the results of the parameters evaluated in quinoa grains, a significant relationship among peak viscosity, breakdown, and setback (0.79) can be found in cold climate conditions, since they result from the interaction among greatest, minimum, and final viscosity. The differential effect presented by the dispersion of cultivars in the inverse relationship between consistency and flow index before and after heating/cooling can be correlated with the dynamics developed by the interaction among starch, protein, and water, and reflect the structural conformation of these macromolecules, so they behave differently as evidenced in productive variables and compositions of different quinoa varieties and under two different agroecological conditions (Gonzalez et al., 2012).
This performance resembles the relationship that exists between amides I and II and protein content with variables such as peak viscosity and setback for most of the quinoa cultivars evaluated, showing that not all cultivars present the same rheological behavior, since both the intensity and functionality of protein secondary structures and starch organization may be encoded particularly by the genetic character of the individual and by the influence of the production environment as reported for wheat cultivars (Yang et al., 2014; Shevkani et al., 2019).
Changes in the functionality of starch present in the grains of different cultivars of the same species are associated with the activity developed by the speed and quantity of isoenzymes synthesized by each species and their interaction with multi-protein complexes that favor mobility in the starch source-landfill, as well as the speed and rate of cutting of branched glucans and derivatives of amylopectin synthesis during grain perisperm formation (Tofiño et al., 2007) that when conjugated with hydric, thermal, or mechanical external processes manifest changes associated with linear or branched chains of glucose and expressed in the techno-functional properties of the matrix (Karwasra et al., 2017).
According to the grouping analysis of the cultivars evaluated (Figure 3), there was no clear discrimination among them. Most of the cultivars were not associated with the place of origin of the grains. However, there is stability in structural composition and rheological performance in the Soracá cultivar (lateral grouping 1), which is associated with the first group of the upper dendrogram and gathers variables such as viscosity peak, breakdown and setback, lipid transmittance, amides I and II, fat content, and consistency after heating/cooling. The trials with the Pasankalla cultivar show a performance preceded by Soracá when grouped in the lateral dendrogram three, although with a greater variation in the evaluated variables’ performance.
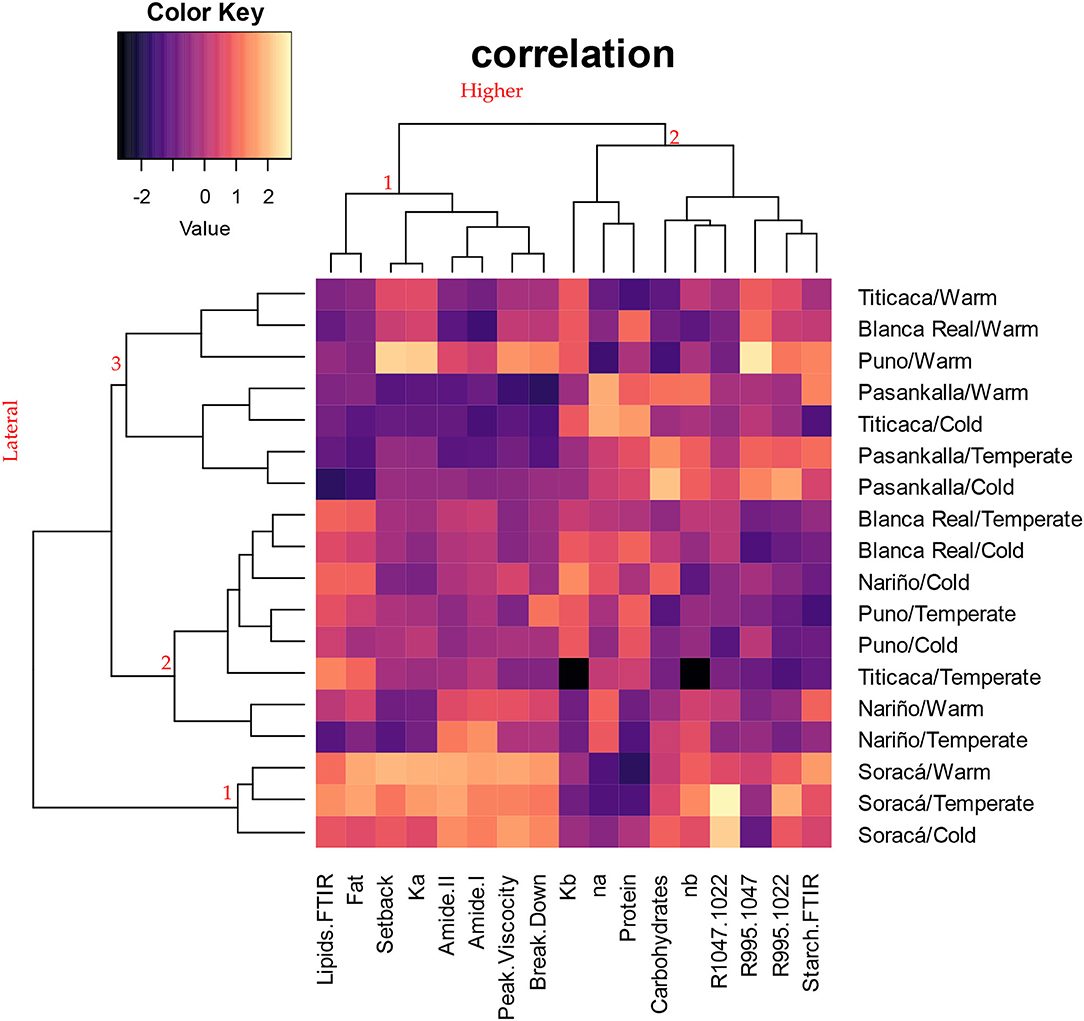
Figure 3. Bootstrap-based clustering analysis with Manhattan distance from different structural and rheological variables for the evaluated cultivars.
Such a phenomenon can be attributed to the effect of ecotypic acclimatization given for these two cultivars being the productive genetic base of the Department of Boyacá and manifested in a lower variability in the compositional and functional characteristics of their grains (Benlhabib et al., 2016).
Conclusions
The results obtained in this investigation support the idea associated with responses that cultivars can manifest as a result of their interaction with photo-thermal and precipitation conditions to determine the content of proteins, carbohydrates and lipids, as well as their structural organization, thus obtaining grains with different potential for use in the food industry by proper selection of cultivars and growth conditions throughout the primary production cycle.
In this sense, despite the fact that the secondary structure of proteins and starch are considered fingerprints for differentiation of the composition of quinoa grains, it is important to highlight that the intensity of peaks is modified by the effect of conditions where the cultivars are established, since this study reports the first advances of the significant differences that the secondary structures present through the FT-IR deconvolution in the intensity of the peaks associated with β-sheet, α-helix and β-turns, Random coil.
In structural organization of starch, bands associated with crystalline and amorphous structures of the grains were significantly changing. However, the 1,075 cm−1 band was shown as a region in the spectrum that is not affected by the two factors evaluated and is proposed as an attribute for monitoring and quality of quinoa products. In this sense, the Soracá cultivar presented the highest ratio of peaks 1,047/1,022 cm−1, allowing films with better gelation and translucent characteristics, while the Puno, Nariño, and Titicaca cultivars presented the lowest values for this ratio, making them important for its incorporation in food matrices where the retrogradation phenomenon is avoided because of low crystallinity index.
From the data, it was possible to find that a satisfactory adjustment to the power model is presented, determining the dispersion flow index of the treatments and facilitating the description of their rheological behavior. In addition, it is evident that the treatments presented a dilatant behavior before heating, but that the dispersion of the treatments presented a pseudoplastic behavior after the thermal treatment. In this sense, the behavior of the consistency index after heating/cooling was relevant, since it was affected by the treatments and was mainly associated with the composition of starch granules in the amorphous and crystalline regions, as well as its relationship with the protein for the formation of gels, generating a marked differentiation between the established treatments.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Author Contributions
MG-P, DR-A, and JB-G: conceptualization. DR-A, MG-P, JH-C, and HV-C: methodology. MG-P and DR-A: software and investigation. MG-P and JH-C: formal analysis. HV-C: resources. All authors have read and agreed to the published version of the manuscript.
Funding
The authors express gratitude to Minciencias’ (Ministerio de Ciencia, Tecnología e Innovación, Before Colciencias) invitation for bid Nr. 779/2017. We are also grateful to the Boyacá Department Government and Universidad del Cauca.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Ahmed, J., Thomas, L., Arfat, Y. A., and Joseph, A. (2018). Rheological, structural and functional properties of high-pressure treated quinoa starch in dispersions. Carbohydr. Polym., 197, 649–657. doi: 10.1016/j.carbpol.2018.05.081
Alandia, G., Rodriguez, J. P., Jacobsen, S. -E., Bazile, D., and Condori, B. (2020). Global expansion of quinoa and challenges for the Andean región. Glob. Food Secur. 26, 100429. doi: 10.1016/j.gfs.2020.100429
Antognoni, F., Potente, G., Biondi, S., Mandrioli, R., Marincich, L., and Ruiz, K. B. (2021). Free and conjugated phenolic profiles and antioxidant activity in quinoa seeds and their relationship with genotype and environment. Plants. 10, 1–15. doi: 10.3390/plants10061046
Bascuñán-Godoy, L., Sanhueza, C., Pinto, K., Cifuentes, L., Reguera, M., Briones, V., et al. (2018). Nitrogen physiology of contrasting genotypes of Chenopodium quinoa Willd (Amaranthaceae). Sci. Rep. 8:17524. doi: 10.1038/s41598-018-34656-5
Benlhabib, O., Boujartani, N., Maughan, P., Jacobsen, S., and Jellin, E. (2016). Elevated genetic diversity in an F2:6 population of quinoa (Chenopodium quinoa) developed through an inter-ecotype cross. Front. Plant Sci. 2:1222. doi: 10.3389/fpls.2016.01222
Bertero, H. D., De La Vega, A. J., Correa, G., Jacobsen, S. E., and Mujica, A. (2004). Genotype and genotype-by-environment interaction effects for grain yield and grain size of quinoa (Chenopodium quinoa Willd.) as revealed by pattern analysis of international multi-environment trials. Field Crop Res. 89, 299–318. doi: 10.1016/j.fcr.2004.02.006
Cantillo, E., and Gracia, M. (2013). Diversidad y caracterización flor?stica de la vegetación natural en tres sitios de los cerros orientales de Bogotá D.C. Colombia Forestal. 16, 228–256. doi: 10.14483/udistrital.jour.colomb.for.2013.2.a08
Curti, R., Sanahuja, M., Vidueiros, S., Curti, C., Pallaro, A., and Bertero, H. (2019). Oil quality in sea-level quinoa as determined by cultivar-specific responses to temperature and radiation conditions. J. Sci. Food Agric. 100, 1358–1361. doi: 10.1002/jsfa.10092
Delgado, A., Palacios, J., and Betancourt, C. (2009). Evaluación de 16 genotipos de quinua dulce (Chenopodium quinoa Willd.) en el municipio de Iles, Nariño (Colombia). Agron. Colomb. 27, 159–167.
García-Parra, M., García-Molano, J., and Deaquiz-Oyola, Y. (2019). Physiological performance of quinoa (Chenopodium quinoa Willd.) under agricultural climatic conditions in Boyaca, Colombia. Agron. Colomb. 37, 160–168. doi: 10.15446/agron.colomb.v37n2.76219
García-Parra, M., Roa-Acosta, D., García-Londoño, V., Moreno-Medina, B., and Bravo-Gomez, J. (2021). Structural characterization and antioxidant capacity of quinoa cultivars using techniques of FT-MIR and UHPLC/ESI-Orbitrap MS spectroscopy. Plants 10:2159. doi: 10.3390/plants10102159
García-Parra, M., Zurita-Silva, A., Stechauner-Rhoringer, R., Roa-Acosta, D., and Jacobsen, S. -E. (2020). Quinoa (Chenopodium quinoa Willd.) and its relationship with agroclimatic characteristics: A Colombian perspective. Chilean J. Agric. Res. 80, 290–302. doi: 10.4067/S0718-58392020000200290
Gonzalez, J., Konishi, Y., Bruno, M., Valory, M., and Prado, F. (2012). Interrelationships among seed yield, total protein, and amino acid composition of ten quinoa (Chenopodium quinoa) cultivars from two different agroecological regions. J. Sci. Food Agric. 92, 1222–1229. doi: 10.1002/jsfa.4686
Granado-Rodríguez, S., Vilariño-Rodríguez, S., Maestro-Gaitán, I., Matías, J., Rodríguez, M. J., Calvo, P., et al. (2021). Genotype-dependent variation of nutritional quality-related traits in quinoa seeds. Plants 10, 1–22. doi: 10.3390/plants10102128
Guerrero-López, A. (2018). Impacto del Cultivo de la Quinua (Chenopodium quinoa Willd) Como Alternativa Productiva y Socioeconómica en la Comunidad Indígena Yanacona de La Vega, Cauca, Colombia. Tesis de Doctorado En Agroecología, Universidad Nacional de Colombia, 133.
Haeberli, W., and Alean, J. (1985). Temperature and accumulation of high-altitude firn in the Alps. Ann. Glaciol. 6, 161–163. doi: 10.3189/1985aog6-1-161-163
Karwasra, B., Gill, B., and Kaur, M. (2017). Rheological and structural properties of starches from different Indian wheat cultivars and their relationships. Int. J. Food Prop. 20, S1093–S1106. doi: 10.1080/10942912.2017.1328439
Labuschagne, M., Geleta, N., and Osthoff, G. (2007). The influence of environment on starch content and amylose to amylopectin ratio in wheat. Starch 59, 234–238. doi: 10.1002/star.200600542
Lee, D. W., Lee, S. K., Rahman, M. M., Kim, Y. J., Zhang, D., and Jeon, J. S. (2019). The role of rice vacuolar invertase2 in seed size control. Mol. Cells 42, 711–720. doi: 10.14348/molcells.2019.0109
Lemoine, R., Camera, S., La, R., Dédaldéchamp, F., Allario, T., Pourtau, N., et al. (2013). Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 24:272. doi: 10.3389/fpls.2013.00272
Lesjak, J., and Calderini, D. (2017). Increased night temperature negatively affects grain yield, biomass and grain number in chilean quinoa. Front. Plant Sci. 8:352. doi: 10.3389/fpls.2017.00352
Li, Z., Chu, S., Wang, P., Gao, S., Li, S., and Yu, X.. (2020). Effects of irradiation treatment on protein structure and digestion characteristics of seed-watermelon (Citrullus lanatus var.) kernel protein. Food Sci. Biotechnol. 29, 1201–1211. doi: 10.1007/s10068-020-00777-9
Manjarres-Hernández, E. H., Arias-Moreno, D. M., Morillo-Coronado, A. C., Ojeda-Pérez, Z. Z., and Cárdenas-Chaparro, A. (2021). Phenotypic characterization of quinoa (Chenopodium quinoa Willd.) for the selection of promising materials for breeding programs. Plants 10:1339. doi: 10.3390/plants10071339
Matías, J., Rodríguez, M., Granado-Rodríguez, S., Cruz, V., Calvo, P., and Reguera, M. (2022). Changes in quinoa seed fatty acid profile under heat stress field conditions. Front. Nutr. 9:820010. doi: 10.3389/fnut.2022.820010
Miranda, M., Vega-Gálvez, A., Martínez, E., López, J., Marín, R., Aranda, M., et al. (2013). Influence of contrasting environments on seed composition of two quinoa genotypes: nutritional and functional properties. Chilean J. Agric. Res. 73, 108–116. doi: 10.4067/S0718-58392013000200004
Nawrocka, A. (2013). Conformational changes in wheat gluten after using Ag-nanoparticles. Int. Agrophys. 28, 311–317. doi: 10.2478/intag-2014-0021
Pezzotti, G., Zhu, W., Chikaguchi, H., Marin, E., Boschetto, F., Masumura, T., et al. (2021). Raman molecular fingerprints of rice nutritional quality and the concept of Raman barcode. Front. Nutr. 8:663569. doi: 10.3389/fnut.2021.663569
Polo, M. P., Roa, D. F., and Bravo, J. E. (2021). Rheological properties of quinoa (Chenopodium quinoa Wild) flours obtained by abrasive milling and heat treatment. Inform. Tecnol. 32, 53–64. doi: 10.4067/S0718-07642021000600053
Präger, A., Munz, S., Nkebiwe, P., Mast, B., and Graeff-Hönninger, S. (2018). Yield and quality characteristics of different quinoa (Chenopodium quinoa Willd.) cultivars grown under field conditions in southwestern Germany. Agronomy 8:197. doi: 10.3390/agronomy8100197
Raynaud, S., Ragel, P., Rojas, T., and Mérida, Á. (2016). The N-terminal part of Arabidopsis thaliana starch synthase 4 determines the localization and activity of the enzyme. J. Biol. Chem. 291, 10759–10771. doi: 10.1074/jbc.M115.698332
Reguera, M., Conesa, C. M., Gil-Gómez, A., Haros, C. M., Pérez-Casas, M. Á., Briones-Labarca, V., et al. (2018). The impact of different agroecological conditions on the nutritional composition of quinoa seeds. PeerJ 14, 1–20. doi: 10.7717/peerj.4442
Roa-Acosta, D. F., Bravo-Gómez, J. E., García-Parra, M. A., Rodríguez-Herrera, R., and Solanilla-Duque, J. F. (2020). Hyper-protein quinoa flour (Chenopodium Quinoa Wild): monitoring and study of structural and rheological properties. LWT 121, 1–7. doi: 10.1016/j.lwt.2019.108952
Ruiz, K. B., Biondi, S., Oses, R., Acuña-Rodríguez, I. S., Antognoni, F., Martinez-Mosqueira, E. A., et al. (2014). Quinoa biodiversity and sustainability for food security under climate change. A review. Agron. Sustain. Dev. 34, 349–359. doi: 10.1007/s13593-013-0195-0
Selma-Gracia, R., Llopis, J. M. L., and Haros, C. M. (2020). Development of new starch formulations for inclusion in the dietotherapeutic treatment of glycogen storage disease. Proceedings 53:3. doi: 10.3390/proceedings2020053003
Shevkani, K., Narpinder, S., Ying, C., and Long, Y. (2019). Pulse proteins: secondary structure, functionality, and applications. J. Food Sci. Technol. 56, 2787–2798. doi: 10.1007/s13197-019-03723-8
Smith, M. R., Rao, I. M., and Merchant, A. (2018). Source-sink relationships in crop plants and their influence on yield development and nutritional quality. Front. Plant Sci. 9, 1–10. doi: 10.3389/fpls.2018.01889
Solarte-Montúfar, J. G., Díaz-Murangal, A. E., Osorio-Mora, O., and Mejía-España, F. (2019). Propiedades reológicas y funcionales del almidón procedente de tres variedades de papa criolla. Inf. Tecnol. 30, 35–44. doi: 10.4067/S0718-07642019000600035
Sonnewald, U., Fernie, A. R., Gruissem, W., Schläpfer, P., Anjanappa, R. B., Chang, S. H., et al. (2020). The Cassava Source–Sink project: opportunities and challenges for crop improvement by metabolic engineering. Plant J. 103, 1655–1665. doi: 10.1111/tpj.14865
Stikic, R., Glamoclija, D., Demin, M., Vucelic-radovic, B., Jovanovic, Z., Milojkovic-Opsenica, D., et al. (2012). Agronomical and nutritional evaluation of quinoa seeds (Chenopodium quinoa Willd.) as an ingredient in bread formulations. J. Cereal Sci. 55, 132–138. doi: 10.1016/j.jcs.2011.10.010
Tetlow, I. J., Morell, M. K., and Emes, M. J. (2004). Recent developments in understanding the regulation of starch metabolism in higher plants. J. Exp. Bot. 55, 2131–2145. doi: 10.1093/jxb/erh248
Thiam, E., Allaoui, A., and Benlhabib, O. (2021). Quinoa productivity and stability evaluation through varietal and environmental interaction. Plants 10:714. doi: 10.3390/plants10040714
Tofiño, A., Ceballos, H., and Cabal, D. (2006). Regulación de la biosíntesis del almidón en plantas terrestres: perspectivas de modificación. Acta Agron. 55, 1–10.
Tofiño, A., Romero, H., and Ceballos, H. (2007). Efecto del estrés abiótico sobre la síntesis y degradación de almidón. Una revisión. Agron. Colomb. 25, 245–254.
Torres, J., Vargas, H., Corredor, G., and Reyez, L. (2000). Caracterización morfoagronómica de diecinueve cultivares de quinua (Chenopodium quinoa Willd.) en la sabana de Bogotá. Agron. Colomb. 17, 60–68.
Wang, X., Zhao, R., and Yuan, W. (2020). Composition and secondary structure of proteins isolated from six different quinoa varieties from China. J. Cereal Sci. 95, 1–26. doi: 10.1016/j.jcs.2020.103036
Wolkers, W. F., Bochicchio, A., Selvaggi, G., and Hoekstra, F. A. (1998). Fourier transform infrared microspectroscopy detects changes in protein secondary structure associated with desiccation tolerance in developing maize embryos. Plant Physiol. 116, 1169–1177. doi: 10.1104/pp.116.3.1169
Yang, X., Wu, L., Zhu, Z., Ren, G., and Liu, S. (2014). Variation and trends in dough rheological properties and flour quality in 330 Chinese wheat varieties. Crop J. 2, 194–200. doi: 10.1016/j.cj.2014.04.001
Yu, X., Yu, H., Zhang, J., Shao, S., Zhou, L., Xiong, F., et al. (2015). Comparison of endosperm starch granule development and physicochemical properties of starches from Waxy and Non-Waxy Wheat. Int. J. Food Prop. 18, 2409–2421. doi: 10.1080/10942912.2014.980949




